LEARN MORE
Inorganic Chemistry
INORGANIC CHEMISTRY STEREO CHEMISTRY IN MAIN GROUP COMPOUNDS
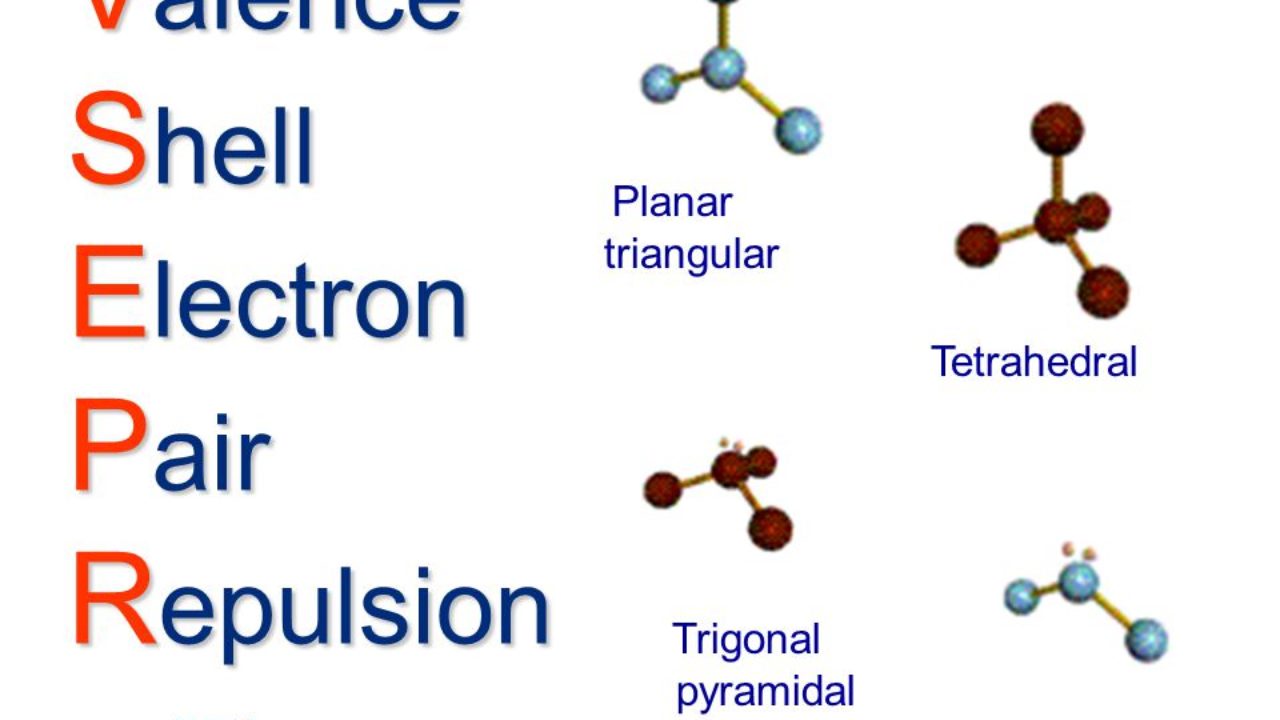
The concept of molecular shape is of the greatest importance in inorganic chemistry for not only does it affect the physical properties of the molecule, but it provides hints about how some reactions might occur. Pauling and Slater (1931) in their Valence Bond Theory (VBT) proposed the use of hybrid orbital, by the central atom of the molecule, during bond formation. Thus, with the knowledge of the hybridisation used by the central atom of the molecule, one can predict the shape and also the angles between the bonds of a molecule. However, since then more advanced theories have come into existence. In this chapter we explore some of the consequences of molecular shape in terms of VSEPR theory and refine that concept into the powerful concept of molecular symmetry and the language of group theory, using Walsh diagrams and Bent's Theory, towards the end of the unit.
You may recall what you have already studied about the directional property of a covalent bond and the concept of hybridisation of orbital to predict molecular geometry.
Starts: May 1, 2020
- H-1006
- Starts:
